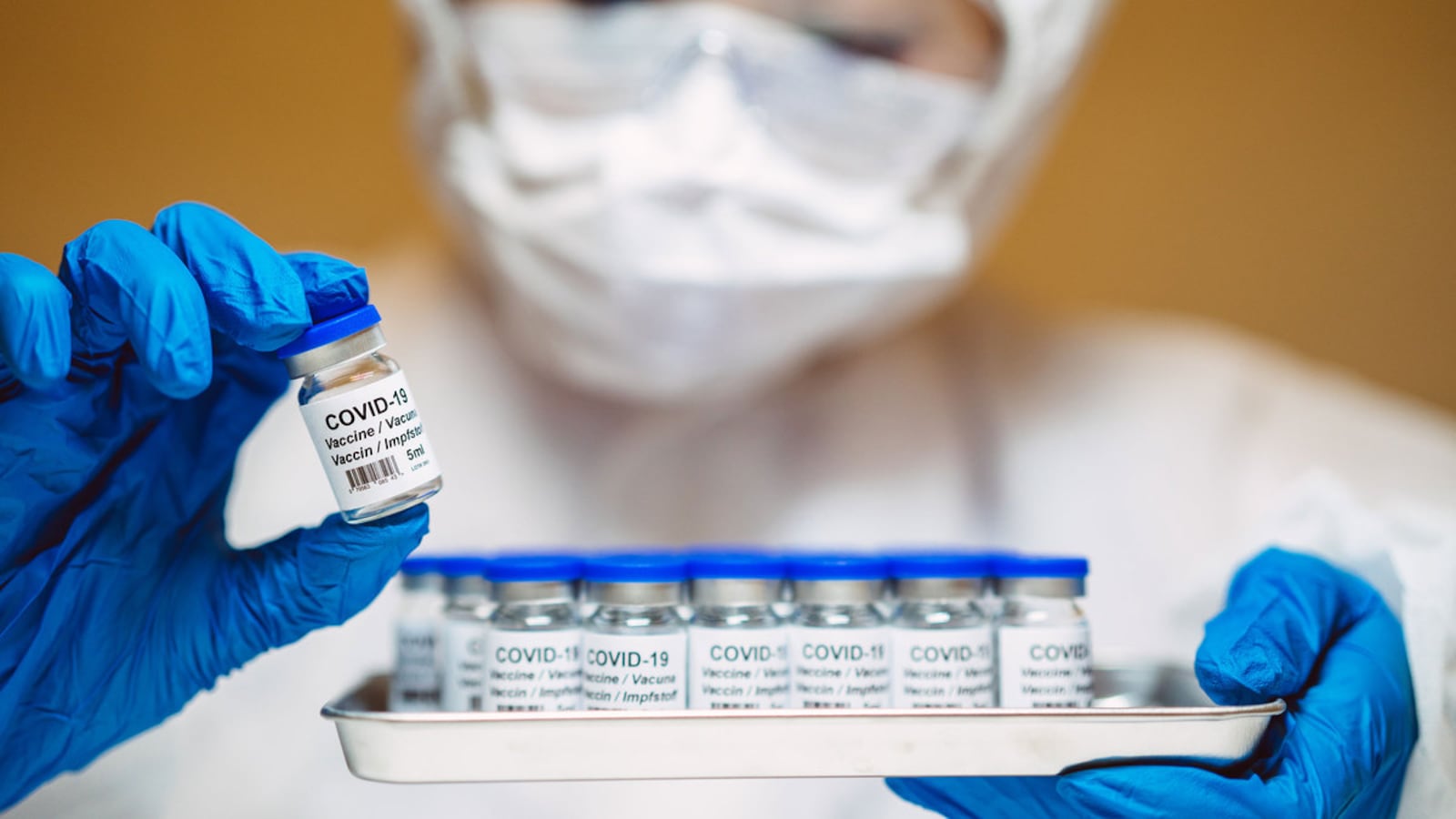
As the pharmaceutical industry inches closer to (hopefully) producing a safe and effective vaccine for the novel coronavirus, everything from the science of the inoculation to how to distribute vaccines that may require cold storage remains in doubt.
But it’s one thing to develop a vaccine and plot how you get it out to the masses, and quite another to actually manufacture doses by the millions. That’s especially true of the leading vaccines from Pfizer and Moderna, both of which rely on cutting-edge messenger-RNA technology.
Welcome to Rabbit Hole, where we dive deep on the biggest story. It’s for Beast Inside members only. Join up today.
Government approval of the manufacturing process isn’t a foregone conclusion, Pfizer CEO Albert Bourla warned in an open letter on Friday. After all, U.S. Food and Drug Administration inspectors will have the final say. “If we achieve a positive efficacy readout and a robust safety profile, the last requirement will be the submission of manufacturing data that demonstrates the quality and consistency of the vaccine that will be produced,” Bourla wrote.
If the FDA doesn’t like what it sees, it could send Pfizer or any other vaccine-maker back to the drawing board to revamp its factories. And that could delay, by many months, the high-stakes global effort to begin immunizing people against the coronavirus.
It’s too risky to cut corners on the factory floor.
“Vaccines can be well-designed, but they have to be manufactured precisely in the same form they were tested, and with the same formulation that was safe and effective,” Jennifer Reich, a University of Colorado sociologist who studies immunization, told The Daily Beast. “Manufacturing must also be free of all contamination that might occur.”
Quality breakdowns have happened before. In 1955, Cutter Laboratories in California accidentally introduced live virus into doses of a polio vaccine it was producing. About 120,000 people got dosed, and 40,000 got sick. Fifty-one people were paralyzed, and five died.
There’s never been an mRNA vaccine before—and that could elevate the risk. This type of vaccine works by introducing a genetically engineered nanoparticle that encourages the production of coronavirus-fighting antibodies into the immune system.
To manufacture a vaccine using mRNA technology, New York-based Pfizer has had to upgrade its factories, including a sprawling, 1,300-acre facility in Kalamazoo, Michigan. The changes could introduce errors into the production process, potentially resulting in weak or ineffective doses.
“We’ve made changes as necessary to ensure we can deliver a potential vaccine,” a Pfizer spokesperson told The Daily Beast. “This includes upgrading site infrastructure to manage ultra-low temp products, expanded capabilities for our distribution centers, and developing a path forward to reach all global destinations.”
Moderna did not respond to a request for comment.
Even with the latest equipment, the process of producing an mRNA vaccine is a tricky one.
“The upstream production processes may involve a number of complexities, including the presence of one or more enzymes, a DNA template for making many copies of the mRNA, etc.,” Cynthia Liu, a scientific information manager with the Ohio-based Chemical Abstracts Service, told The Daily Beast. “The mRNA molecule may also need to be wrapped in a nanoparticle to prevent mRNA breakdown by enzymes in the human body before it gets into human cells. The final formulation also needs to be optimized to enhance the effectiveness and stability of the vaccine.”
In plain language, that means the factories have to copy tiny biological particles, then surround them in a protective casing of fat. The resulting vaccine doses are unstable, however. You have to freeze them immediately—and keep them frozen until it’s time to administer them.
It’s delicate and difficult work, and the pharmas and the FDA need to be sure factory staff can pull it off. “The production process must be optimized to ensure they can efficiently and consistently account for these needs and generate that same product repeatably, at scale,” Liu said.
The FDA has had a process for checking the production quality of any new vaccine—one that predates mRNA vaccines. After all, older factories producing less advanced vaccine types also face their own production challenges. “The quality and consistency issue is not unique to an mRNA vaccine,” Liu explained.
A thorough production quality-control check is the final bureaucratic hurdle before the FDA will approve a vaccine for widespread use. The agency first assesses a vaccine-candidate for safety and effectiveness. Then the agency takes a hard look at the manufacturing process.
For coronavirus vaccines, the FDA will be paying particular attention to “history and qualification of cell banks, history and qualification of virus banks, and identification of all animal-derived materials used for cell culture and virus growth,” the agency explained in June guidance to vaccine-makers.
In addition to reviewing production data from the pharmas, the FDA sends inspectors to vaccine factories all over the world so they can see for themselves. The FDA’s process “assures the identity, strength, quality and purity of drug products,” Surinder Singh, a vice president at Japan-based pharma Otsuka Pharmaceutical, told The Daily Beast.
Henry Wang, a chemical engineering professor who studies vaccine production at the University of Michigan, said that Bourla’s warning notwithstanding he expected Pfizer’s doses to be “well-developed and -manufactured.”
And the company, like other vaccine makers, is already churning out doses, anyway. “Pfizer has been investing at risk since the early days of the pandemic to perfect our manufacturing processes and rapidly build up capacity,” Bourla wrote. The company wants to have millions of doses ready to ship if and when the FDA gives it the thumbs-up.
But that strategy could backfire—not only for Pfizer and other manufacturers, but also for the feds as well as the millions of Americans eagerly awaiting a vaccine.
As Reich put it, “If the clinical trials fail to show that a vaccine is effective and safe, all those doses of the failed vaccine would need to be destroyed.”