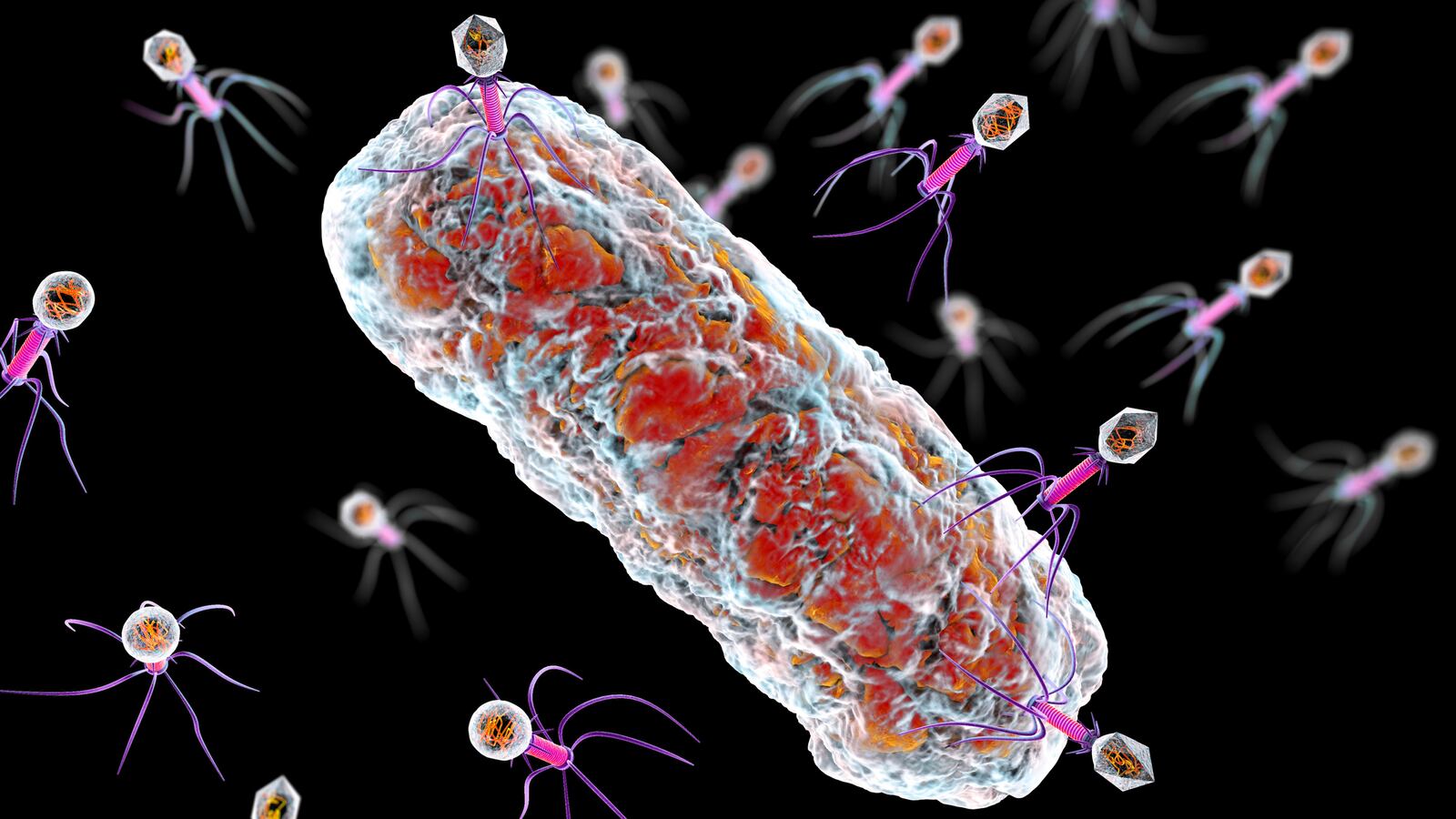
The apex predator of the microbial world is a type of virus called bacteriophages. It hunts down and kills bacteria by hijacking the microorganism’s reproductive machinery and tearing it apart. Because of this, scientists have commandeered these microscopic killers to fight on the frontlines of a growing threat: antibiotic resistance, or when bacteria find ways to overcome the drugs meant to stop them.
A 2019 global survey found more people died from antibiotic resistance than HIV/AIDS or malaria. We've even seen a precipitous rise of drug-resistant gonorrhea, Salmonella, among many other diseases in recent years. Equally worrisome is an uptick in drug-resistant Mycobacterium chelonae—a bacteria similar to the ones behind diseases like tuberculosis and leprosy—threatening people who are immunocompromised, and a major concern in public health.
That’s where bacteriophages come in. Researchers in Boston have used a therapy using the virus to successfully treat a man suffering from drug-resistant M. chelonae infection, according to a new study published Tuesday in the journal Nature. The infection belongs to a group of mycobacteria called nontuberculous mycobacteria, considered the most drug-resistant of the bunch—and, therefore, one of the most difficult to treat.
“There is a long history of bacteriophage therapy for a variety of bacterial infections,” Dr. Jessica Little, an internist at Brigham Women’s Hospital and the study’s first author, told The Daily Beast in an email. “This [study] is the first case of phage therapy for the treatment of a particular organism and the first case of a single bacteriophage rather than a cocktail [of bacteriophages] used for the treatment of nontuberculosis mycobacteria.”
This type of bacteria is typically found in water and soil. On the whole, they’re pretty harmless—that is, until these microorganisms enter the human body through contaminated drinking water or a breach in the skin. The nasty pathogen causes serious infection in the skin, soft tissues (like cartilage, fat, muscle, and tendons), and even the lungs, according to NYU Langone Health. It doesn't affect everyone, but if you do have an underlying health condition like a weakened immune system, you’re at a much greater risk. For those patients, conventional antibiotics aren’t very helpful since mycobacteria are stubbornly resistant, rapidly developing ways to subvert modern medicine.
But that doesn’t mean it's undefeatable. In the past, scientists have used engineered bacteriophages to treat lung infections caused by Mycobacterium abscessus, which inspired Little and her team to investigate whether the same could be used to treat the bacteria’s close relative M. chelonae.
The key to this is finding just the right bacteriophage. Like a picky eater, bacteriophages have certain bacteria they like to feast upon. Luckily, scientists developed centralized libraries and databases of the microbes. One such effort has been through the Science Education Alliance-Phage Hunters Advancing Genomics and Evolutionary Science (SEA-PHAGES) initiative, which has so far isolated and cataloged more than 20,000 phages found in nature.
Little and her team sent a sample of M. chelonae collected from their patient, an 45-year-old man with the auto-immune disease rheumatoid arthritis, to Graham Hatfull at the University of Pittsburgh whose lab jointly oversees SEA-PHAGES and also studies mycobacteria bacteriophages. After rounds of testing, one promising candidate arose: a phage called Muddy.
Armed with the right phage, the researchers grew lots of it and gave it to their patient through an IV drip. The man wasn't improving while on antibiotics and even after undergoing surgery to remove his infected skin lesions caused by M. chelonae. But within months of getting Muddy pumped into his veins, the man’s painful skin lesions shrunk, eradicating the bacteria without any negative side effects.
This is a promising first for treating specific antibiotic-resistant infections with a specific bacteriophage rather than a whole spectrum of bacteriophages, which is how the therapy is typically given to bolster its bacteria-killing power. The wholesale approach can allow the bacteria to actually become phage resistant. However, Little cautioned that this is just one case study with one patient. “Clinical trials are needed to better understand the benefits of phage therapy on a larger scale and in a more controlled setting,” she said.
Scientists also don’t have a complete picture of how bacteriophage therapy works at the immune-system level and the potential risks. So far, clinical trials with human patients have been safe overall with not the many negative side effects reported. But it’s not easy to locate the right phage for the right disease. It’s also challenging to manufacture the therapy on a large commercial scale. However, Little believes that it's crucial that researchers build off of the research to help improve treatments for the most vulnerable patients out there.
“Antimicrobial resistance is a serious and increasing threat globally and phage therapy is a promising new therapeutic option for the treatment of these infections,” said Little. “There are an incredible number of different phages that can treat unique bacteria—this is precision medicine and it is complicated—but I believe that the development of non-antibiotic, pathogen-focused strategies to complement the tools we already have is very important at this moment.”