The day parents all over the world have been waiting for—after nearly two years of the pandemic—is finally here.
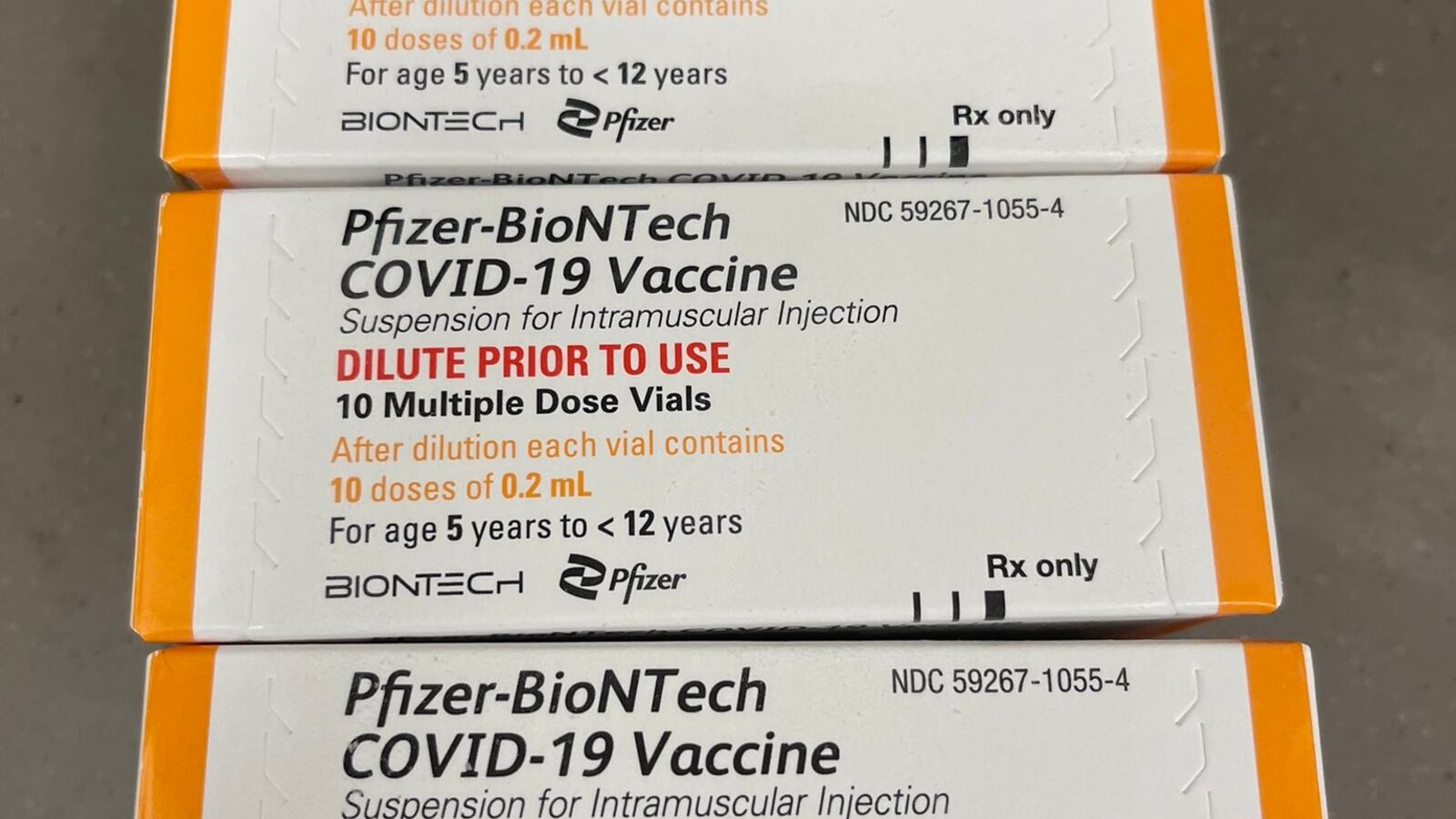
Dr. Rochelle Walensky, the Director of the Centers for Disease Control and Prevention, has authorized the distribution of the Pfizer-BioNTech COVID-19 vaccine to 5- to 11-year-olds. Her signature officially green-lit pediatric jabs at the state level, which could begin in a matter of days.
“Together, with science leading the charge, we have taken another important step forward in our nation’s fight against the virus that causes COVID-19, We know millions of parents are eager to get their children vaccinated and with this decision, we now have recommended that about 28 million children receive a COVID-19 vaccine," Walensky said. "As a mom, I encourage parents with questions to talk to their pediatrician, school nurse or local pharmacist to learn more about the vaccine and the importance of getting their children vaccinated.”
Walensky's sign-off was the last major regulatory step in the authorization process for Pfizer vaccines going to young children. It came hours after an independent panel of experts on the U.S. CDC's Advisory Committee on Immunization Practices had unanimously recommended the vaccination, in a 14-0 vote.
The committee's decision came after it concluded the benefits of the vaccine outweighed its risks, which include rare cases of heart inflammation linked to the Pfizer and Moderna jabs.
“We have one more vaccine that saves the lives of children,” Dr. Sarah Long, a pediatrician and member of the panel, said after the vote. “We should be confident to use it to the maximum.”
In the past, Walensky has typically adopted ACIP’s recommendations, though not always. In September, she “shocked” some of the panel when she recommended Pfizer vaccine booster shots for frontline workers, going against the committee’s advice.
However, at the beginning of the panel's meeting, Walensky's remarks indicted her thinking. “Today is a monumental day in the course of this pandemic,” she said, noting that the risk from COVID-19 to children has been “too high and too devastating” to ignore.
“There are children in the second grade who have never experienced a normal school year,” Walensky added. “Pediatric vaccination has the power to help us change all of that.”
The Biden administration’s pandemic-response coordinator, Jeffrey D. Zients, had said earlier on Tuesday that the U.S. Pfizer vaccination program for children ages 5 to 11 would be “running at full strength” as of the week of Nov. 8.
There are 28 million children aged 5 to 11 in the United States. The age bracket makes up 9 percent of all coronavirus cases in the U.S., and nearly 40 percent of all pediatric cases.
Doran Fink, clinical deputy director of the FDA’s vaccine division, said the COVID case rate among young children in the 5-to-11 group is currently “near the highest” of any demographic. In the past week alone, per an American Academy of Pediatrics report, more than 100,000 children tested positive for COVID-19.
Pfizer has said that in clinical trials on young children, their two-dose vaccine was “safe, well tolerated, and showed robust neutralizing antibody responses.” Their jab, which has one-third the dose that older children and adults receive, was deemed 90.7 percent effective in protecting against symptomatic coronavirus.
The company submitted its data to the Food and Drug Administration in late September, aiming to obtain emergency-use authorization.
Last Tuesday, considering Pfizer’s findings, an FDA panel voted to endorse the new, lower dose of the vaccine for young children. The recommendation was passed to the desk of FDA Acting Commissioner Janet Woodcock, who authorized it Friday.
Zients noted that, minutes after the FDA’s Friday authorization, the government began shipping dose vials of the Pfizer-BioNTech vaccine for young children to distribution centers. The process started with 15 million doses, which will be parceled out from the centers to approximately 20,000 pharmacies, family doctors, and pediatricians around the U.S..
“More doses will be packed and shipped and delivered each and every day over the next week,” Zients said.