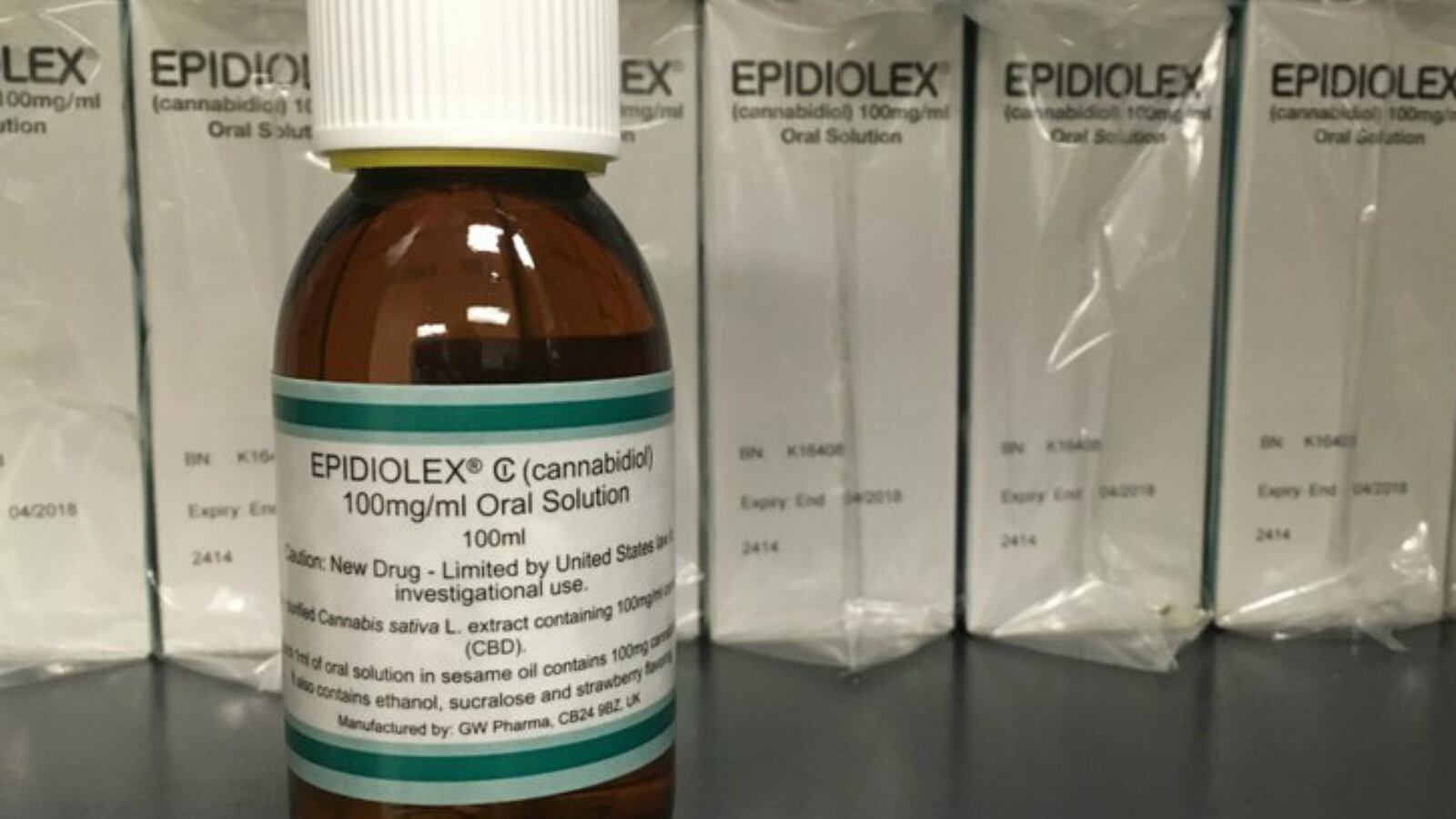
A 13-member FDA panel voted “unanimously in favor” of an experimental medication with a cannabis chemical to treat “severe seizures in children with epilepsy” on Thursday, according to the Associated Press. Epidiolex, a syrup formulated by GW Pharmaceuticals, is essentially a “pharmaceutical-grade” CBD oil—which does not have the “mind-altering” effects that THC does. If the FDA follows the group's recommendation to approve the drug, it would be the “first drug derived from the cannabis plant” to get the agency’s approval in the U.S. Some patients involved in the drug’s trials had dozens of seizures a day—and said it dramatically reduced the number of seizures they experienced, allowing them to live a normal life. The panel backed the trial up with three studies that show how the drug has helped with “two rare forms of childhood epilepsy.” It’s not clear why the substance reduces seizures.
Read it at Associated Press