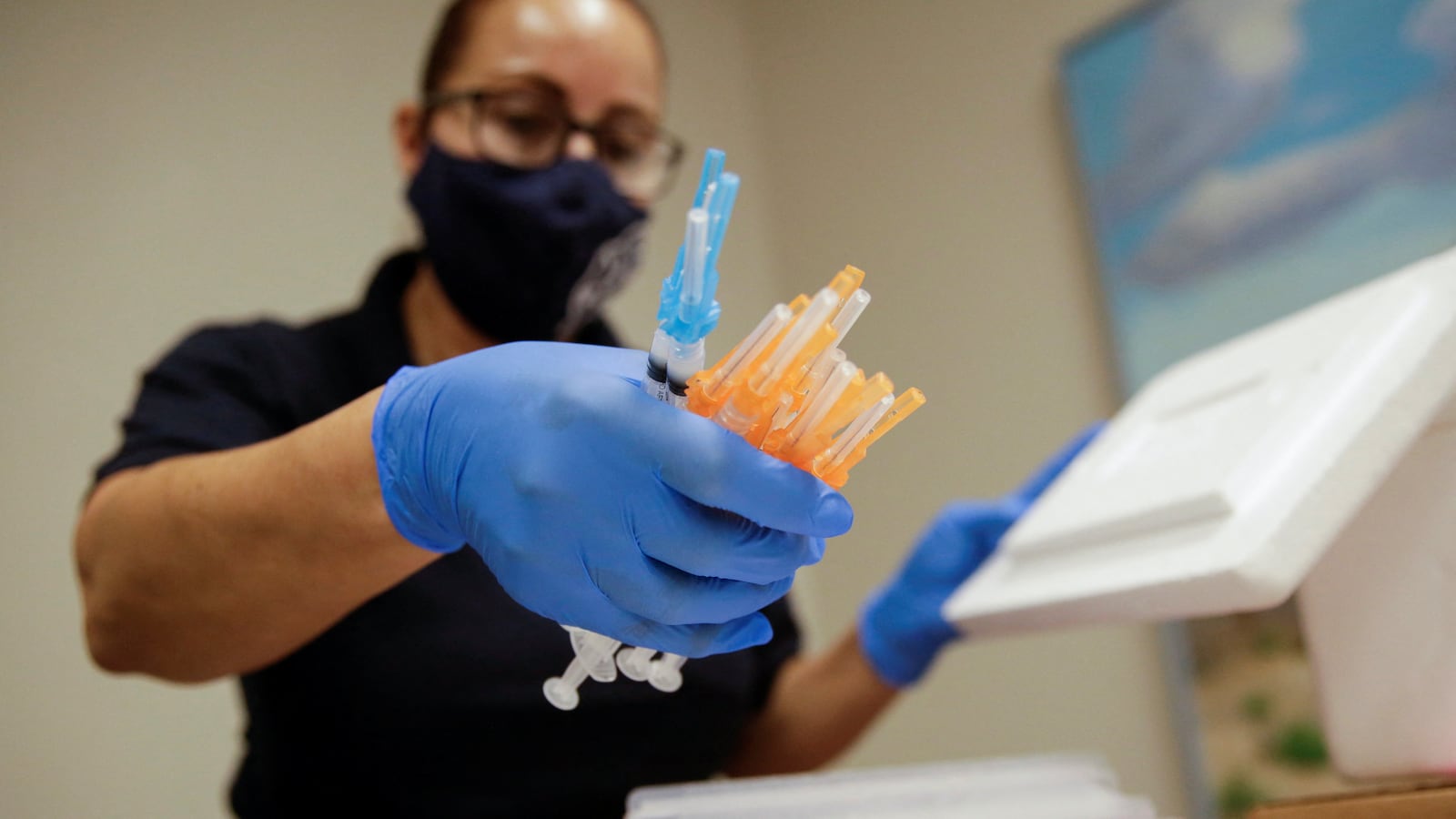
The U.S. Food and Drug Administration gave the green light on Monday to newly formulated COVID-19 vaccines from Pfizer-BioNTech and Moderna. The new boosters target widely circulating offshoots of the Omicron variant that have caused a recent spike in cases and hospitalizations nationwide. The next step in rolling the shots out will be a Tuesday meeting by an independent advisory panel from the CDC, which is expected to provide recommendations on who should be eligible to take them. After the CDC’s director gives the final sign-off, the vaccines will be shipped out—and could be available as early as next week. “The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality,” Dr. Peter Marks, an FDA official, said in a statement. “We very much encourage those who are eligible to consider getting vaccinated.”
Read it at The New York Times