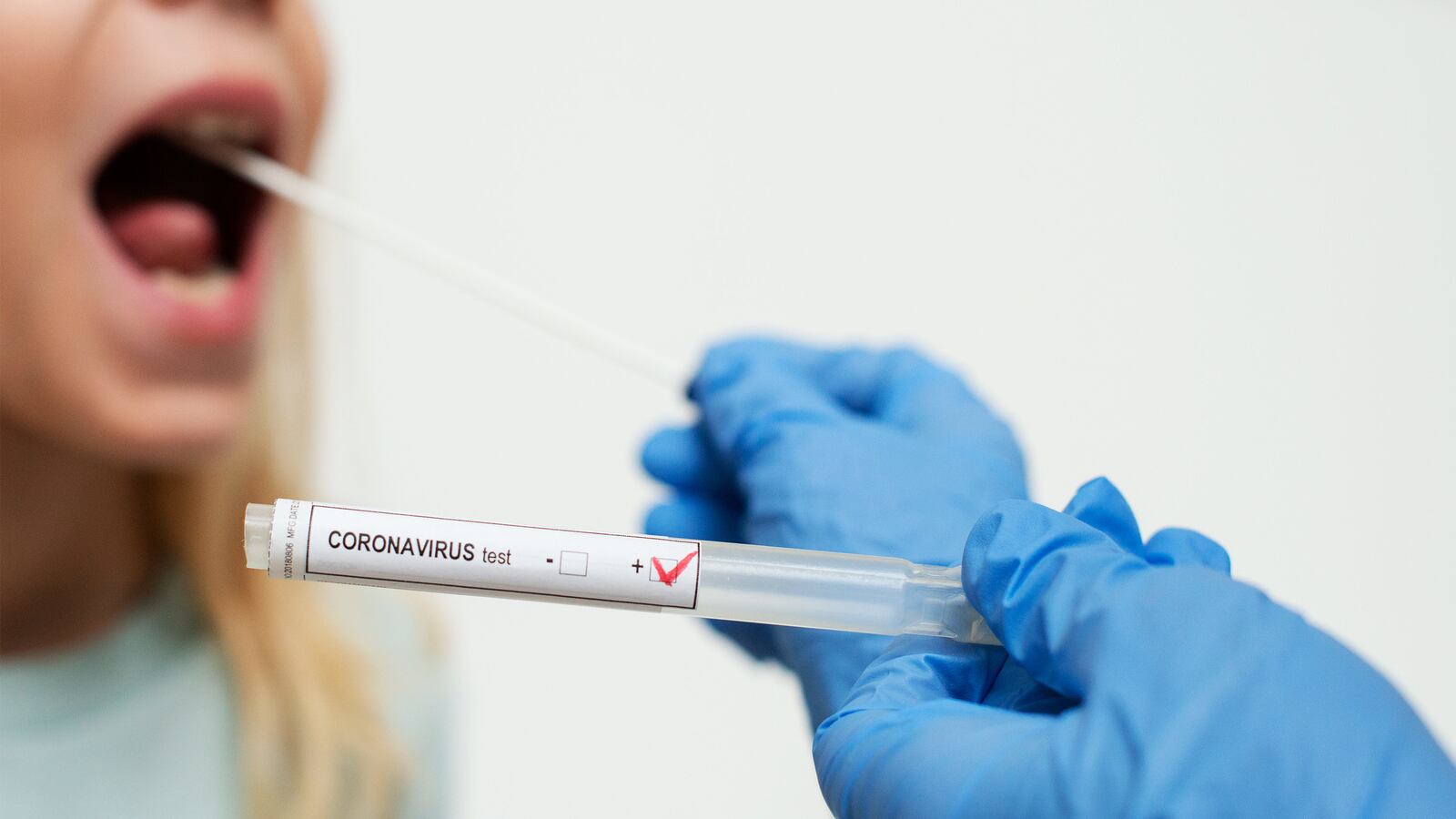
As states grapple over how and when to restart their economies, health experts have agreed on one thing above all else: better COVID-19 testing capacity will be critical for quickly identifying and responding to new cases and minimizing the coronavirus threat.
But a powerful shortcut to ramping up the number of Americans who get tested may be sitting in plain sight.
New studies have begun raising the profile of a longstanding testing strategy for infectious diseases as a way to dramatically increase capacity while minimizing America’s notorious bottleneck of shortages in lab supplies. It’s called sample pooling, or pooled testing, and proponents say it could go a long way toward filling the country’s glaring gaps in COVID-19 surveillance.
“I’m surprised that not all people are doing it,” said Baha Abdalhamid, assistant professor of pathology and microbiology at the University of Nebraska Medical Center in Lincoln.
The gist here is simple. It involves pooling, or combining, a portion of the nasal swab or saliva-based samples taken from multiple patients—from four to 30 people or even more in some cases—into a single tube for one COVID-19 test. A negative test result in that case offers a high degree of confidence that everyone in that pool was COVID-19 negative, and would spare health departments from having to follow up with each individual. A positive test result would require the retesting of samples from each individual within the pool.
In theory, a relatively low rate of COVID-19 within the test population would yield enough negative results to slash the overall number of tests needed for widespread surveillance and follow-up diagnoses. Of course, a higher rate of positive cases—as in, say, a hot zone like New York—might not lend itself as readily to the method.
Isaac Bogoch, an infectious diseases specialist at Toronto General Hospital and the University of Toronto, said pooling was a “smart approach” that could save both time and money if done properly. “It’s a good strategy, it’s been used in the past, you can do terrific surveillance quickly and then home in like a laser beam if you need to, and it’s usually a cost-effective approach,” he told The Daily Beast.
Public health workers have used the method for years to screen populations for other viral diseases like HIV and Hepatitis B and C; Bogoch and colleagues used it to successfully screen schoolchildren in Ivory Coast for a parasitic flatworm infection called schistosomiasis. The next phase of the COVID-19 pandemic response, he argued, will require lower barriers to diagnostic testing, rapid turnaround time, and the ability to respond faster to test results. Anything making that process easier, he said, “Is definitely a step in the right direction.”
Most COVID-19 diagnostic tests are based on a well-established lab technique called polymerase chain reaction, or PCR. Like an office copier that can churn out multiple copies of a report, every cycle of a PCR reaction creates additional copies of all of the RNA present in a test sample. Eventually, the reaction generates so many copies that the presence of the unique genetic signature for the SARS-CoV-2 virus can be detected by probes designed to stick to that telltale sequence of RNA.
The pooling method’s usefulness, experts say, depends in part on the lower limit of RNA at which the PCR test can still detect copies of the virus; this is because the pooling process effectively dilutes the genetic material. The strategy also depends on the rate of false-positive and false-negative test results, and on the overall prevalence of disease in a tested population. The higher the prevalence, the lower its helpfulness, though current rates suggest many states could benefit.
A recent proof-of-concept study led by Abdalhamid affirmed the technique’s potential to dramatically increase COVID-19 testing capacity and slash use of lab reagents, the testing chemicals that have been hard to come by as the pandemic has accelerated, in a population with a disease prevalence of about 5 percent.
For the study, his research group used a web-based application to calculate an optimal pool size of five patient samples each, based on a known positivity rate at the time of about 5 percent among all tested samples in Nebraska. To assess their strategy, the group constructed 25 pools—each with a known positive sample and four negative samples. The PCR test revealed the presence of the virus in every case. The test also successfully picked out two positive results from a group of 60 patient swabs combined into 12 pools of five samples each.
Based on the study’s promising results, the U.S. Food and Drug Administration allowed the lab to modify its CDC-developed diagnostic kit under an emergency use authorization. On March 24, the Nebraska Public Health Lab, which is located within the medical center, began pooling some of its incoming patient samples. Through the end of April, Abdalhamid said the lab had combined about 5,600 samples into 1,120 pools and saved a significant amount of time and at least 50 percent of its testing reagents.
“It’s definitely a faster turnaround for us with the pooling,” he said. “Not only can we test more patients, but we are also saving a lot of money.”
If every test consists of five pooled patient samples, for example, a lab could run five times as many tests at the same time. Positive results would require individual follow-ups, of course, but a reasonably low rate of them would permit labs to run significantly more tests during any given period. Decreased lag time and a faster negative test result for a suspected COVID-19 case, in turn, could shorten the duration of a patient’s time in quarantine and reduce the need for other prevention control measures.
Stanford University scientists took a different tack by using a pooled sampling method to detect community transmission of COVID-19 in the San Francisco Bay Area. The researchers tested roughly 2,900 patients with upper respiratory symptoms by grouping the samples into pools of nine or 10. Of the 292 initial tests, two pools came back positive for COVID-19, while the researchers reported one additional false-positive result. Additional tests on individual samples from the flagged pools confirmed the presence of the virus in exactly two cases. Those two individuals had contracted COVID-19 in mid-February, the study found, retroactively adding to the state’s early tally.
Researchers at Saarland University Medical Center in Hamburg, Germany recently developed their own, similar, strategy and reported successfully pooling of up to 30 samples per initial COVID-19 test, with what they deemed “sufficient” diagnostic accuracy. In their analysis, they screened 1,191 patient samples with only 267 tests to yield positive results for 23 individuals—less than one-fourth the number of tests that would normally be required. A separate U.S. collaboration also concluded that the method could help point out community clusters for targeted public-health interventions.
In another recent study co-authored by Roy Kishony, a professor of biology and computer science at Technion – Israel Institute of Technology in Haifa, researchers detected a single positive sample for SARS-CoV-2 in pools of up to 32 samples. But they had an estimated false negative rate—that is, a failure to detect true cases—of 10 percent. In an email to The Daily Beast, Kishony said his group measured significantly lower false negative rates in smaller pools of 8 to 16 samples.
Israel, Kishony said, has been actively developing the pooling strategy, particularly at Haifa’s Rambam Hospital, where collaborators worked with him and a Technion colleague to develop the method. So far, the group has been approached with requests for help and advice from more than 10 countries on five continents. India has already begun its own pooled testing. Similarly, Abdalhamid has heard from interested public health officials in Singapore and Puerto Rico.
But the technique hasn’t yet caught on in much of the U.S. The Daily Beast reached out to 12 state and county health departments—a mix of hot-spots and locales gearing up to reopen—to inquire about their plans. Most did not respond prior to publication, while state health departments in Hawaii, Minnesota, and Tennessee said they weren’t planning to pursue pooled testing as a strategy.
A spokesperson for the Minnesota Department of Health expressed reservations in an emailed statement, citing concerns about the testing method’s lower sensitivity and usefulness in areas with higher COVID-19 prevalence. The health department’s lab, she said, “is operating in low-risk tolerance in that we don’t feel comfortable losing sensitivity in our testing.”
The spokesperson noted that pooled testing can be useful in screening thousands of samples for a very rare condition, as is commonly done in blood banks. “We are responding to outbreaks where we already expect to find a high prevalence, so it would not be a good option for the testing we are doing at this time,” she said. According to data collected by the COVID Tracking Project, Minnesota had a relatively high positivity rate of about 14 percent, on average, over the past week.
A spokesperson for the New York City Department of Health and Mental Hygiene, though, said the agency had discussed the pooling strategy with the team of Nebraska researchers. Although not currently using the method due to widespread transmission in New York, the spokesperson said, “It is certainly something we will consider when the positivity rate is low enough.”
For pooling to be efficient, Kishony conceded, tested populations should have a positive COVID-19 rate of less than 10 percent—and ideally close to 1 percent. (Other epidemiologists have cited an upper cutoff rate ranging from 5 to 15 percent.)
“In many places, current positive rates for symptomatic patients could be too high for efficient pooling,” Kishony added. But the method could be far more suitable for routine screening of specific populations, such as factory shift workers, hospital staff, and army units. A positive pooled test result, Kishony said, could trigger immediate safety precautions even in the absence of identifying individual patients.
Abdalhamid said he and his colleagues were conducting a follow-up study to evaluate whether they can make their pooling strategy even more efficient by grouping patients into high risk, low risk and unknown risk categories, based on their symptoms. The researchers won’t pool any tests from high-risk patients, given the expectation that many will come back positive. Instead, they’re reserving the pooling scheme for patients in the low and unknown risk categories. The researchers were still crunching the numbers to determine whether their modified strategy might pan out, he said.
Either way, pooling could help health officials across America gain new ground on an elusive disease. What remains to be seen is how willing they are to try it.