As coronavirus infections skyrocket and hospitals across the United States fill up with serious COVID-19 cases, the U.S. Food and Drug Administration this week approved the first-ever rapid home COVID-19 test for emergency use.
The All-In-One Test Kit from California biotech firm Lucira Health uses some of the most reliable molecular-RNA technology, takes just 30 minutes, and is more than 90 percent accurate, according to the company. The FDA hopes the kits will result in more people getting tested for the coronavirus, and health experts cheered the development—even as they warned about the potential for individuals to botch the process outside of a medical setting.
Welcome to Rabbit Hole, where we dive deep on the biggest story. It’s for Beast Inside members only. Join up today.
“A test that can be fully administered entirely outside of a lab or healthcare setting has always been a major priority for the FDA to address the pandemic,” Jeff Shuren, director of FDA’s Center for Devices and Radiological Health, said in a statement late Tuesday. “Now, more Americans who may have COVID-19 will be able to take immediate action, based on their results, to protect themselves and those around them.”
Health experts welcomed the FDA’s emergency-use authorization as long lines at testing sites around the country make the rounds on social media during the pandemic’s so-called Third Wave. “You have to start somewhere and this seems like a good somewhere,” David O’Connor, a vaccine researcher at the University of Wisconsin-Madison, told The Daily Beast.
But experts also warned that greater access to testing is no panacea. The Lucira home test requires a prescription and, for a while at least, could be in short supply. And without widespread contact-tracing and quarantining, there are limits to how much good testing can do, regardless of how accessible it is. As Susan Butler-Wu, a clinical microbiologist at the University of Southern California, previously told The Daily Beast, “We can’t test our way to normalcy.”
The FDA’s nod clears Lucira to sell at-home test kits to doctor’s offices and pharmacies. Doctors can use the kits themselves on patients 13 and older, or prescribe them to patients 14 and older, according to the FDA’s approval guidelines.
The $50 kits will be available in Florida and California in the coming weeks before rolling out to health-care providers nationwide in early 2021. Lucira announced it’s also aiming to set up a mail-order system, whereby people can consult with a doctor online and then get a test kit in the mail for home use.
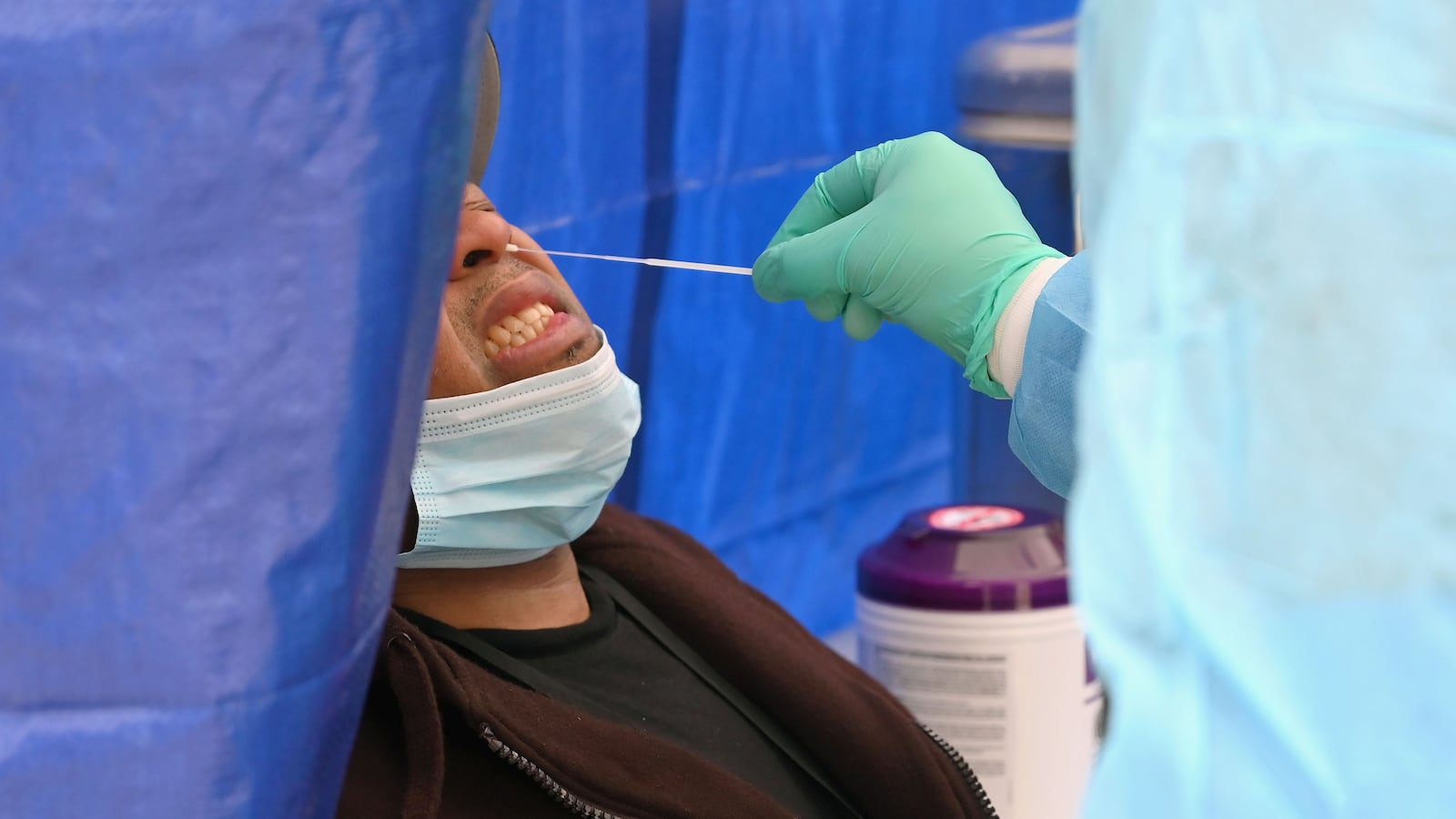
Using the kit is straightforward. You swipe a swab in your nostril, swirl the swab in a vial containing a special solution, then plug the vial into a fist-size analysis device. After half an hour, the device flashes “positive” or “negative.” A positive result means the user should immediately self-isolate and contact their doctor for treatment options.
There are potential flaws in the system, as O’Connor pointed out. A good deep swabbing is critical to the test’s functioning—and some people might be squeamish about sticking a swab that far up their own nose. O’Connor also expressed doubts about the test’s sensitivity; Lucira admitted that the test may have failed to detect “very low levels of virus that possibly no longer reflected active infection.”
“Possibly” is the key word here. Lucira did not immediately respond to a request for comment.
Then there’s the reporting problem in a country where contact tracing has been a miserable failure. “How will positive and negative results get reported?” O’Connor asked. Under-reporting could distort local responses to outbreaks.
Ideally, a doctor or nurse would supervise at-home tests remotely via video-chat and check in on the results the same way, Butler-Wu said.
But at present, there’s no government requirement for that kind of supervision. And while there is a requirement for doctors to report new COVID cases, there’s no way to compel the actual users of an at-home test to tell their own health-care providers when they’ve tested positive. “Somebody might not be forthcoming with results,” Butler-Wu warned.
There are obvious reasons for people to lie about having COVID, O’Connor explained. Many employers require infected workers to stay home. But not all employers continue paying employees when they have to skip work. Local, state, and federal support for COVID-positive workers is uneven and, in many places, hard to access. For many people, “a positive test result means a loss of income,” O’Connor said.
Reporting gaps aside, initial authorization of at-home testing is an encouraging development. “The fact remains we have large swathes of the country where people are not able to get test results back rapidly,” Butler-Wu said. The Lucira test could put a small dent in that huge problem.
“Baby steps are still steps in the right direction,” O’Connor added.
Perhaps the best thing about the FDA giving Lucira the green light for home-testing is that it signals wider progress across industry toward faster, cheaper, easier, and more accurate testing.
“Lots of other people are working towards at-home collection and testing,” Christopher Mason, a professor of genetics at New York’s Weill Cornell Medicine, told The Daily Beast.
Still, what we really need is reliable, affordable home tests that people can order and use all on their own—now. As Eric Topol, a cardiologist with Scripps Clinic in California, argued on Twitter late Tuesday, that means “without prescription, cheap, [in] every household, ASAP.”