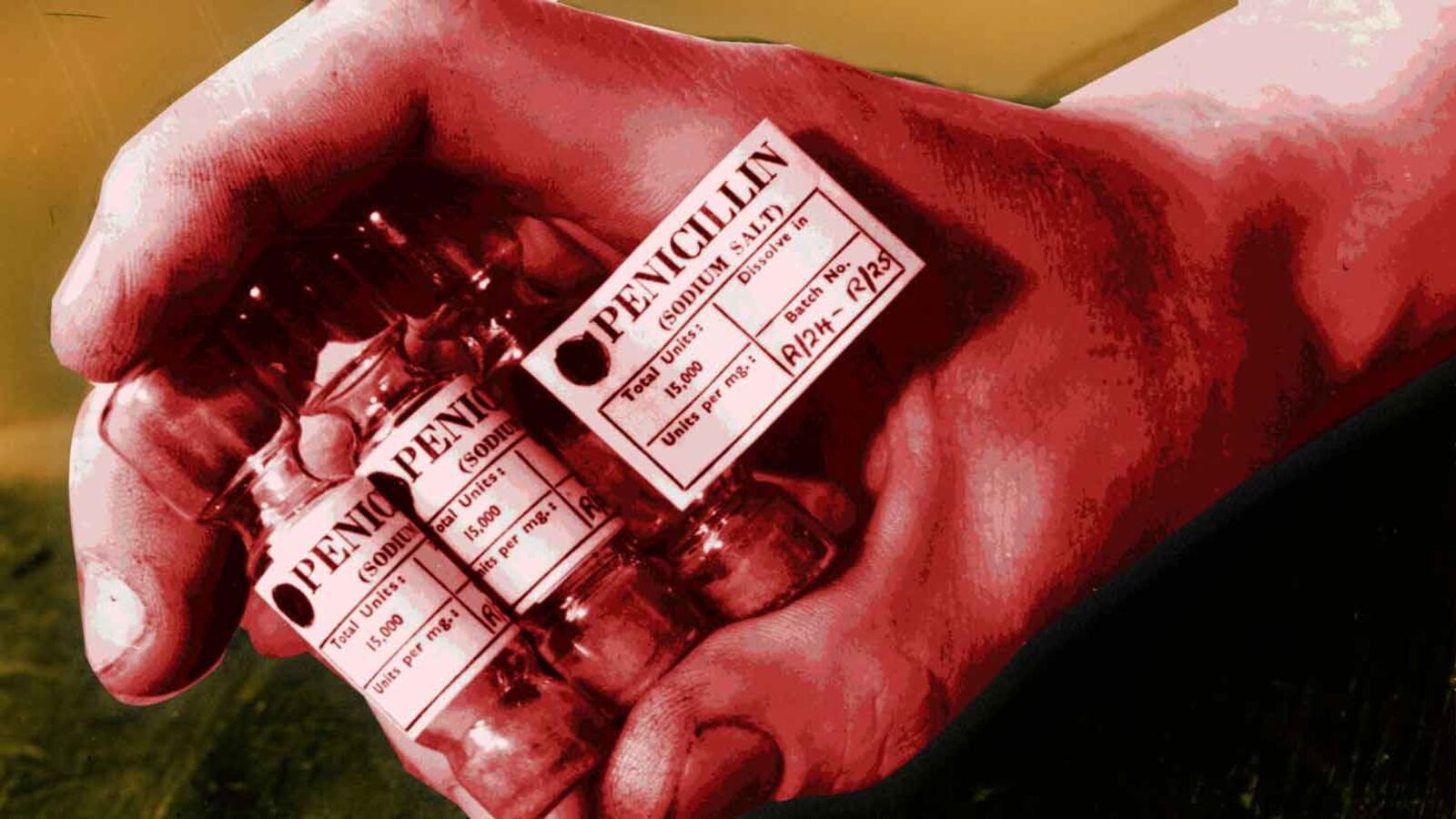
If you say that you’re allergic to penicillin—a narrow-spectrum antibiotic that, for many bacterial infections, is still considered to be a “wonder drug”—your doctor won’t prescribe it. Once you write it on those forms in the waiting room, or tell your pharmacist, “penicillin allergy” becomes part of your permanent medical record.
Yet a growing body of evidence suggests that most people who say they’re allergic to penicillin are, well, wrong. In a recent study published in Journal of Allergy & Clinical Immunology, nearly 90 percent of patients who had “penicillin allergy” listed on their medical charts were found to actually have no such allergy at all.
“There’s this problem—what you could consider an epidemic—of people labeled with unverified penicillin allergy. It’s the number one drug allergy that’s listed in patients’ records,” Dr. Dave Khan, a professor at the University of Texas Southwestern Medical Center and co-author of the study, told The Daily Beast. Over 1 in 10—up to 15 percent—of Americans has a reported penicillin allergy. That’s more than the number of adults in the U.S. who have hay fever (7.8 percent), and the number of children under age three who have food allergies (8.0 percent).

Overusing and misusing the term “allergy” is to blame, Khan said. Many people are misdiagnosed because they mistakenly attribute their symptoms (or their child’s symptoms)—such as a rash (“that looks like measles”), an upset stomach, vomiting, diarrhea, and even hives—as proof of an allergy.
“Some people have so many antibiotic allergies listed that it’s actually hard to pick a drug,” Dr. Faoud Ishmael, who works as a pediatric allergist at the Penn State Hershey Medical Center, added. “It’s because they’re allergic to pretty much every available class of oral medication.”
After penicillin is prescribed for a routine infection—a parent takes a child to the pediatrician for an ear infection, or you schlep to the doctor for a strep throat or sinus infection—you might experience symptoms that look and feel like an allergy.
If you report this to your doctor, and it seems like you might have an allergy, then “penicillin allergy” will likely go on your chart, Ishmael said. Many doctors err on the side of caution when performing a clinical diagnosis, because in the case of a true allergy, which is rare (3 to 10 percent of the population), the most severe (though very rare, at one to 5 in 10,000) reaction is anaphylaxis, which can be life-threatening and requires a trip to the emergency room.

The only way to know if you’re actually allergic to penicillin is a skin test. Khan argues that getting the right diagnosis lowers health care costs and weakens antibiotic-resistant bugs (and infections) for all of us. Knowing the truth won’t just save you money and get you out of the hospital sooner (on average, one day per patient), it also supports antimicrobial stewardship, which is the idea that you should kill bacteria (or slow their growth) with the right size weapon, for the shortest amount of time. But to do that, you can’t just take a patient’s word for granted. Allergies must be tested and verified.
Over the 18-month study, over 250 patients who entered the hospital were prioritized for testing, first by an algorithm that studied electronic health records, and then by a dedicated pharmacist who reviewed patients’ charts. The patients first took a skin test, PRE-PEN (Benzylpenicilloyl polylysine), followed by a “challenge”: 500 mg of amoxicillin, a penicillin-like antibiotic. The patients who were found to be not allergic had the allergy label removed from their records, which had an impact on their care in the hospital. A third of the patients switched to penicillin or cephalosporin, which reduced the use of broad-spectrum antibiotics considerably (e.g. vancomycin by 34 percent, clindamycin by 61 percent). This switch saved an estimated 500 inpatient days, and nearly 650 outpatient days.
“With some medications such as aztreonam [a penicillin alternative], the cost of a day of therapy is much higher than that of beta-lactam antibiotics,” Kristin Alvarez, associate director of Clinical Advancement at Dallas’ Parkland Hospital, said. The screening and testing process takes about two-and-a-half hours per patient, and costs $120 in supplies.
“[We] are about to roll out to the ambulatory setting with group visits,” Alvarez said, though Parkland Hospital has been using the physician-pharmacist protocol for individual patients since October 2014. The team has removed over 500 penicillin allergy labels from the electronic health record system.
The paper concludes that hospitals should use a “physician-pharmacist team model” for allergy testing. That’s a big departure from how allergies are typically dealt with—by a specialist, via a referral from a primary care doctor.
“Ideally, you’re going to test people as soon as they get entered into the hospital. It’s the perfect time to do it,” Dr. Eric Macy, a physician at Kaiser Permanente in San Diego, said. “Just challenge them on admission. The floor nurse watches them for an hour, and if no rash occurs, then they’re not allergic. Take it off their chart. And then, they can get the appropriate antibiotic that day.”

In an editorial in The Journal of Allergy and Clinical Immunology: In Practice with Dr. Werner Aberer, Macy warns that an “unverified” penicillin allergy poses “a significant public health risk.” But, he admitted that it is very common: He estimates his office has treated 6,000 patients in 20 years. He sees it all the time.
Yet in many cases, penicillin is what doctors want to prescribe. Penicillin is still their first choice. “Very usual infections—strep throat, sinusitis, cellulitis—are best treated with penicillins and their relatives,” Dr. Kimberly Blumenthal, a physician at Massachusetts General Hospital, said.
Macy says that if you have “penicillin allergy” on your chart, you’ll likely end up with a broad-spectrum antibiotic, such as clindamycin, or even a third-generation cephalosporin.
Dr. Blumenthal reports that “alternative antibiotics” are less effective, more toxic, and more costly. Many are intravenous only, and carry the potential for side effects.
“This class of antibiotics is associated with a higher risk of opportunistic infections, like clostridium difficle,” said Alvarez.
Patients are also more likely to come in contact with antibiotic-resistant bugs, such as vancomycin-resistant Enterococcus (bacteria that can colonize and infect a number of sites in the body, from the bloodstream to the urinary tract) and methicillin- or vancomycin-resistant Staphylococcus aureus, which can lead to what’s commonly known as a ‘staph infection’ and can be life-threatening.
“We will never solve this problem with just allergists,” Blumenthal said. “Allergists have to let go of this territorial thing that they’ve had for so long, where we’re saying, This is our area. We have to instead say, ‘This is our area to teach the family practitioner to do this safely. It is our area to teach the hospitalist in my mass general to do this correctly.’”