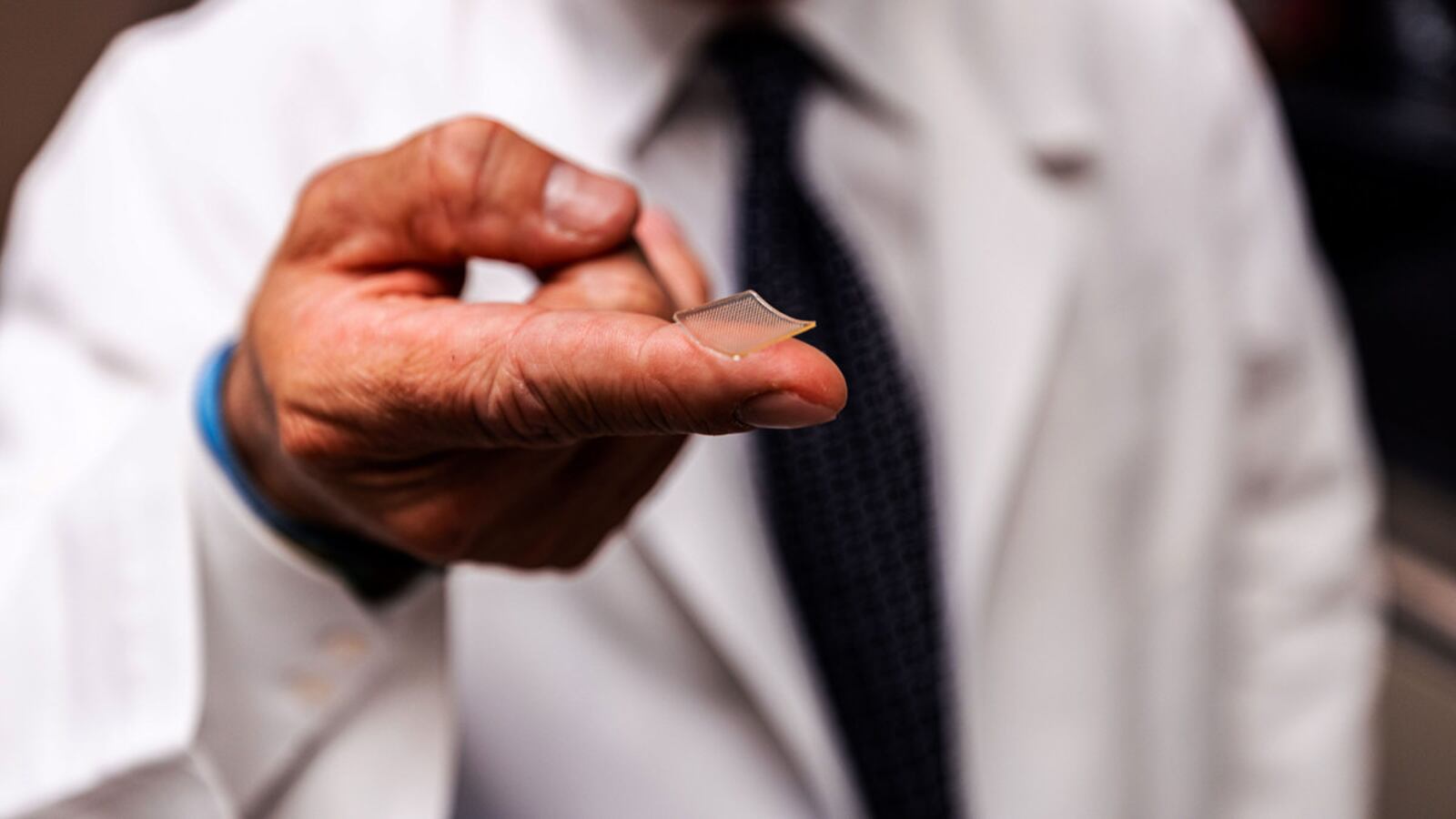
It looks like a band-aid with a bed of microscopic crystal-like spikes underneath. If it’s successful in human trials, the tiny piece of cutting-edge skin velcro developed by researchers at the University of Pittsburgh Medical Center could help put an end to the COVID-19 pandemic. It’s called PittCoVacc and it’s a microneedle vaccine candidate for the novel coronavirus that researchers hope will prove just as effective in developing an immune response in people as it has been in mice.
So what’s a microneedle array? How does it work? And when will we know if it’s effective?
Welcome to Rabbit Hole, where we dive deep on the biggest story. It's for Beast Inside members only. Join up today.
Different kind of vaccine
The PittCoVacc works differently than the kind of inoculations we’re most familiar with. Oftentimes vaccines are made by taking a weakened or dead virus that your body can easily beat up on and build immunity to.
The PittCoVacc is what’s known as a recombinant protein vaccine. Rather than injecting you with a weakened virus, researchers synthesize a specific protein from the virus known as an antigen. That antigen is injected into your body so your immune system can recognize it and develop antibodies that can clobber the infection if it ever encounters an entire virus.
“It’s safer because it uses a much smaller fragment of the actual pathogen,” Dr. Louis Falo, a professor and chair of the UPMC dermatology department who helped research the PittCoVacc, told The Daily Beast.

Tip of the spear
UPMC researchers were able to get a vaccine candidate into animal trials just three weeks after China released the genetic sequence of SARS-CoV-2, as the virus is officially known, to the public. One of the reasons they were able to act so quickly is because UPMC’s previous research on vaccines for Middle East Respiratory Syndrome, a member of the same coronavirus family, helped give them a head start.
While researching MERS inoculations, Falo and his fellow researchers found that using a particular kind of antigen was especially effective in generating antibodies in mouse trials. Viruses like MERS and SARS-CoV-2 use what’s known as a spike protein to find and attach to receptors on your cells and inject them with the virus’s genetic material.
UPMC researchers focused on a tiny piece of the MERS virus’s spike protein, known as subunit S1, when developing a vaccine candidate for that virus.
“From our previous work we learned that there’s a particular component of the S1 protein that more or less serves as a key on the virus to open the lock to allow it to enter human cells,” said Falo. “Our idea was, if we could focus on that area of the key to make an antibody response against, the antibody would block the interaction between the key and the lock and therefore prevent infection.
The tactic had shown promise in previous animal trials on MERS so when the genetic code of the virus that causes COVID-19 was sequenced, the Pittsburgh team went for the same approach. The results in animal trials so far look similarly promising but researchers won’t have a better idea for sure until the vaccine goes into human clinical trials.
Immuno first responder
The way PittCoVacc’s delivers its inoculation is as novel as the way it stimulates immune responses.
Researchers developed what’s called a microneedle array, a small, clear band aid-sized strip made of tiny sugar crystal needles less than half a millimeter thick. The array goes over your skin and reportedly feels like velcro—less painful than a traditional needle stick.
But the delivery method isn’t about soothing the nerves of the needle-phobic. Instead, it’s designed in part to help make the vaccine more effective.
“It enables us to very specifically target an area of the skin which is incredibly immunogenic,” meaning it’s likely to generate an immune response, Dr. Falo explained.
“If you think about it, the skin is our first line of defense. It’s constantly exposed to viruses, bacteria, and other harmful invaders. Because of that, it has evolved a very efficient system for generating immune responses,” said Falo. “That system includes what we would call scout cells or dendritic cells to look for things that don’t belong there and carry pieces back to the rest of the immune system to generate antibody responses against.”
Sugar makes the medicine go down
The PittCoVacc embeds the protein it uses to generate an immune response into the sugar-like structure of the needles so when it’s pushed into the skin, the moisture in your body melts the needles, and the vaccine’s antigen is released into a part of the skin that’s likelier to generate a strong immune response.
Microneedle arrays have been around for a few years but so far they’ve not been used in any large scale production for a vaccine or other drug but Falo says he expects that to change as more researchers and drug makers learn about the technology.
Scale it up
The other advantage of a microneedle array approach to vaccines is that they’re relatively durable and capable of being produced en masse in short order. The crystalline needles used to press the vaccine into your body can be churned out in molds and the cells designed to churn out spike protein fragments can be manufactured at scale.
One of the biggest problems for distributing vaccines in the developing world is the lack of reliable electricity and cooling technology that can store vaccines requiring refrigeration. If it’s successful in human trials, the PittCoVacc would alleviate those problems as it can be stored at room temperature.

Delivery date
Since a vaccine is the only thing that can bring the COVID-19 pandemic to a close once and for all, the biggest question on everyone’s mind is: when can we get it? For PittCoVacc, the short answer is that researchers can’t quite say when yet.
Vaccines take time to develop for a lot of reasons. There’s the regulatory requirement of making sure that vaccine candidates that look promising in the lab are safe to test on humans and then there’s the time it takes to see if those human subjects actually develop an immune response and stay healthy.
UPMC researchers have already gone through animal trials with their mouse research but right now they’re working with the FDA to ensure that their vaccine candidate is safe for human trials.
Once they’re through that hurdle, the time frame for when a vaccine could be approved, if proven effective, will become clearer. “As we start getting results in from the clinical testing, that will dictate the length of time it will take overall,” said Dr. Falo.