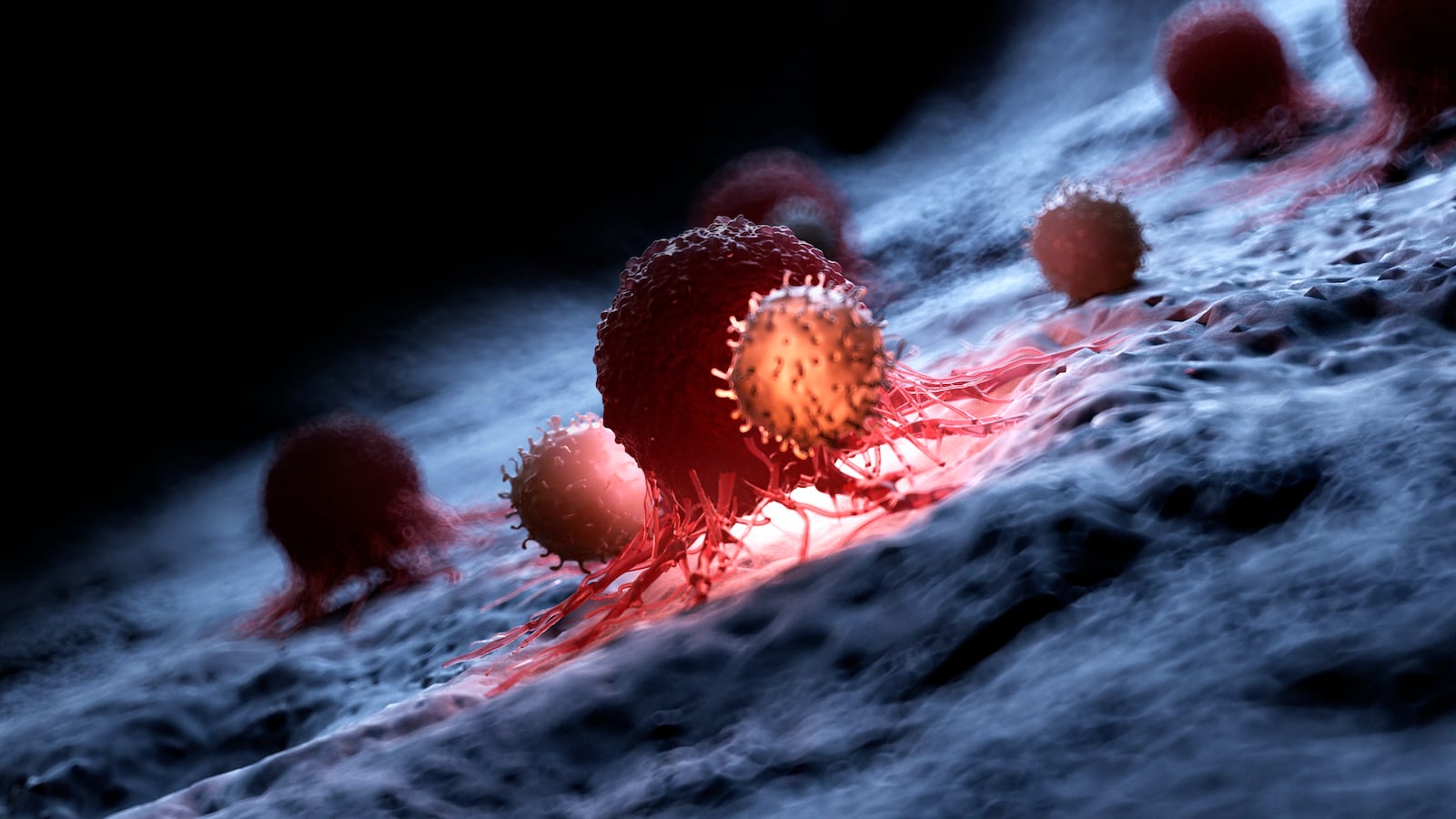
There’s a security force of nearly a billion immune cells—called T cells—patrolling the inside of your body. Its job is to look out for any suspicious-looking characters, like a nasty cold virus, and get rid of them. They're also useful in the fight against cancer by way of an innovative treatment called chimeric antigen receptor (CAR) T-cell therapy.
Unlike conventional cancer treatments that rely on cancer-killing drugs or radiation, this process takes a patient’s own T-cells and genetically engineers them to track down and kill tumor cells. But one major problem with this treatment is that you can’t take these engineered T cells and use them for someone else since they run the risk of getting rejected, similar to organ transplants. Luckily, scientists might have found a workaround that might end up saving cancer patients money as well.
Researchers at Toronto General Hospital and the University of Toronto have developed a therapy that uses a double-negative T cell, a unique type that manages not to trip any alarm bells in your immune system and get rejected when transplanted. In a new study published on Friday in the journal Science Immunology, these CAR-T cells were effective in fighting cancer in Petri dishes and in mice with transplanted tumors derived from human tissues. Most importantly, these cells weren’t rejected, a revolutionary finding that could make using CAR-T cells a more effective and accessible therapy for many cancer patients.
T cells looks for other cells carrying antigens that don’t resemble any found in the body. It latches onto them and initiates the foreign cell’s self-destruct sequence, where toxic chemicals are secreted inside the cell and kill it. Using this method, the cells can target viruses and bacteria. But cancer is wily and through mutations, it can bypass a T cell’s detection by pretending to be a healthy cell or suppressing the immune system entirely.
What CAR T-cell therapy does is help the immune system overcome this deception. To make this treatment, T cells are harvested from the blood of a donor—typically the patient—and a new gene that teaches it how to recognize a tumor cell is introduced. These engineered T cells have shiny, new antigen receptors (a protein on the cell’s surface that recognizes cancer cell proteins) and are then grown in a lab. Once there are enough of them, usually in the millions, the CAR T-cells are put back into the patient and start targeting and killing cancerous cells.
CAR-T cell therapy is a great option for treating advanced leukemias and lymphomas, keeping the cancers at bay for many years—but it comes with a downside: graft versus host disease (GvHD). The condition, commonly seen with organ transplantation, is triggered when donated cells are recognized are rejected by the patient’s body, resulting in autoimmune symptoms, Li Zhang, a cancer researcher at the University of Toronto and the new study’s lead researcher, told The Daily Beast.
Zhang has spent a little over a decade trying to figure out a way to make a more effective version of the therapy and came across a cell scientists have known for years but have somewhat overlooked: double-negative T cells, a rare subset that lacks the proteins that can cause this rejection.
“We’ve developed these double-negative T cells for many years, starting with a mouse model study, and then about 12 years ago, we moved to generating human double-negative T cells,” she said. “Over the years of research, we realized that double-negative T cells, unlike conventional T cells, won’t cause GvHD when you inject them into [mouse cancer models].”
This got Zhang and her co-author, Jong Bok Lee, an immunologist at Toronto General Hospital Research Institute, thinking about adapting it for treating cancer. They took double-negative T cells from a healthy human donor and inserted a gene that helps it recognize a protein that tends to be elevated in certain types of lymphomas and leukemias. The researchers found that the engineered T cells were effective in eradicating cancer but also didn’t trigger GvHD in mice.
While Zhang and Lee are planning to conduct clinical trials in the future, this finding holds a lot of promise. It means there’s a way to avoid GvHD while also creating a universal treatment that is available to virtually any patient.
It’s also cost-effective. A single CAR-T cell treatment can cost upwards of thousands and dollars so the overall hospital bill for a patient can be exorbitant. Zhang and Lee see the possibility of their new CAR T-cell therapy being cheaper via mass production and potentially something patients can pick up off the shelf of a pharmacy.
“We can scale them up and generate large batches,” Lee told The Daily Beast. “We could make them an off-the-shelf product where the patient can come in and purchase it without paying millions of dollars for a life-saving drug.”