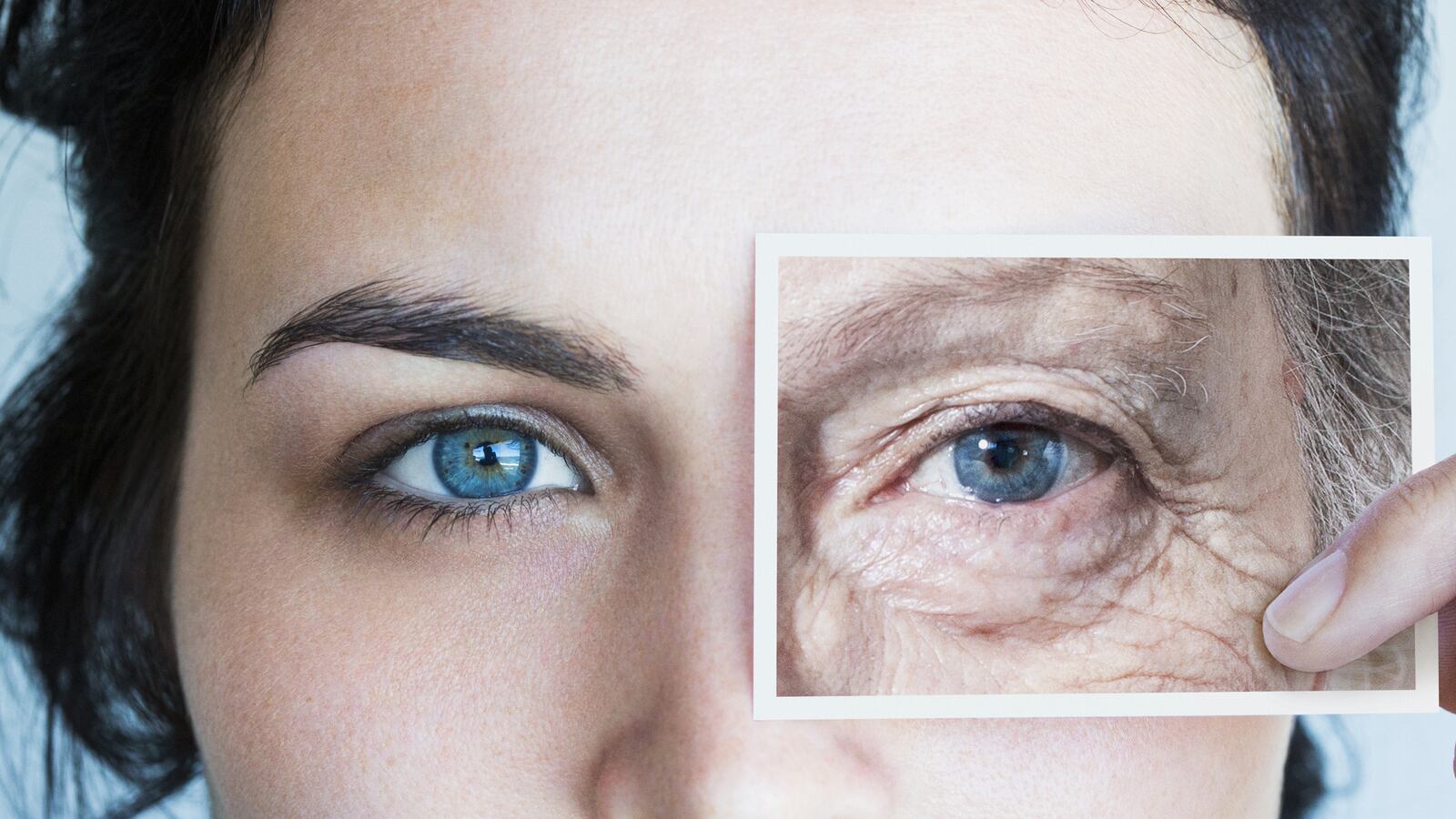
It has long been claimed that being “young at heart” is the key to retaining your physical vigor—new evidence suggests that is true. Three studies by researchers at Stanford and Harvard suggest the age of the blood flowing through your heart could radically alter how young you feel.

The bottom line is that scientists have just recently discovered that transfusing older mice with the blood of younger mice could combat certain age-related diseases. To understand the importance of these results, it is critical to understand the background of these experiments and the questions that the researchers were looking to answer. Is there an innate, yet dormant capacity within the elderly to actually reverse their ailments if only given the right signals?
Stem cells. Many cells in the human body retain the ability to grow and divide, producing identical replicas of themselves. However, there are some cells, termed stem cells, that retain some ability to differentiate into a number of different types of cells. Stem cell differentiation involves a plethora of regulatory factors and signals that are in a constant state of flux. A leading hypothesis in aging is that stem cells become less effective over time—consider the extended recovery times in elderly Americans or the nagging injuries that plague older athletes. Many of us have noticed “We’re not 20 anymore.” While these slower healing times are likely multifactorial, these new findings support a significant role for stem cells in what we think of as the effect of aging.
Scientists previously demonstrated that the process of aging did not rid tissue of its stem cells. Therefore, these researchers set out to identify the signals, potentially carried in the blood, that could reinvigorate the dormant stem cells of elderly mice. In other words, maybe the hardware was in place, but the software was not taking advantage of it. These studies began with a technique called parabiosis, which involves stitching the flanks of two mice together so that blood vessels of the two mice grow together and eventually give rise to a shared circulatory system. Evidence from a number of studies pointed to a protein called GDF11. Researchers found much more GDF11 in young mice blood and determined that treatment with this protein alone recapitulated some of the effects of the transfusions.
Scientists demonstrated the benefits of these “young blood infusions” in reversing aging effects in three different body systems. In muscles, young blood revived strength and endurance in old mice. In the heart, it reversed age-induced cardiac hypertrophy (enlargement of the heart). Recent findings now point to rejuvenating effects in the brain.
An initial study, by Villeda et al., noted that young blood increased the activity of neural stem cells, which gave rise to new neurons in the hippocampus, the area of the brain responsible for memory consolidation and spatial navigation. The authors followed that initial finding with a more detailed investigation. After injection of whole, young blood into elderly mice, they found changes in gene expression indicating changes in synaptic plasticity (changes in the communication between neurons), differences in the structure of neurons themselves, and even measurable cognitive improvement in the treated mice. The old mice with young blood injections could better navigate mazes and remember fear memories when compared to control animals.
These remarkable findings hold potential in the study of various musculoskeletal, cardiac, and cognitive challenges posed by aging. However, waking up dormant stem cells requires caution.
These studies should serve as evidence of the critical value of biomedical research and of the wealth of knowledge that remains unexplored. Stem cell biology may very well hold the answers to aging, along with a host of other medical challenges such as cancer, traumatic brain injury, stroke, and even wound healing.
A note of caution must be sounded, however. Stem cells usually give rise to new cells under strict regulatory processes within the body. When those regulatory signals are modified, as in the case of GDF11 in these studies, unwanted proliferation can occur. This unwanted cell replication is more commonly known as cancer.