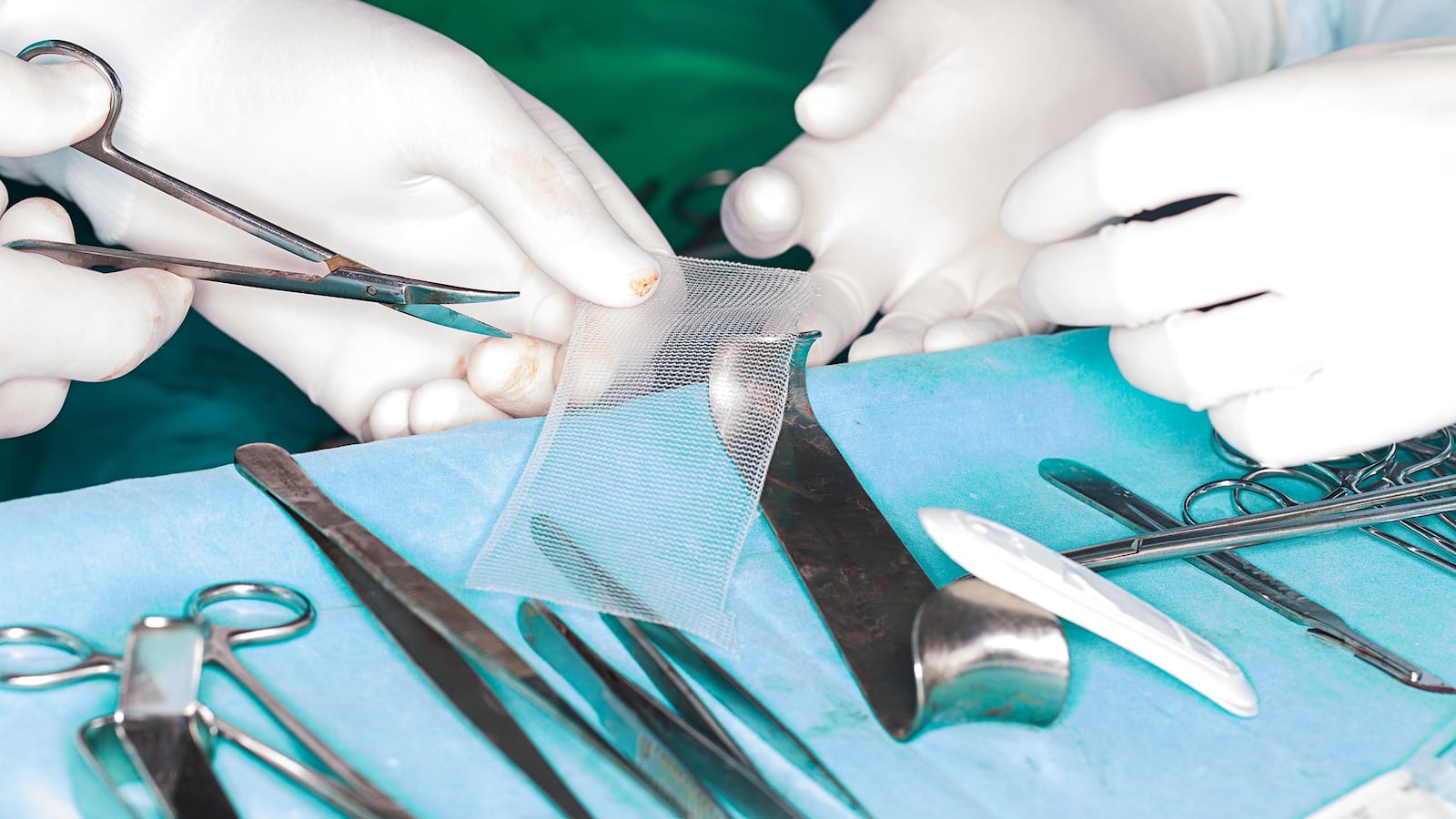
In the last two decades, millions of women have turned to vaginal mesh implants as a possible solution to two annoying problems: urinary incontinence and pelvic organ prolapse, where body parts shift out of place due to childbirth or the ageing process.
But as tens of thousands of lawsuits illustrate, the simple device wasn’t the miracle many were sold on. Some women say the mesh left them in chronic pain, caused infection, and interfered with their sex life.
And now, an investigation published by the British Medical Journal suggests that problems with the implants—plastic or surgical mesh shaped into a supportive hammock and placed in the body under local anesthesia—go back to the beginning and are clouded by allegations of payment and questionable studies.
The implant was invented by Swedish obstetrician Ulf Ulmsten, who signed a licensing agreement in 1997 with Johnson & Johnson subsidiary Ethicon.
The first round of tests conducted by Ulmsten had an incredible result: 84 percent of women cured of incontinence within two years. But it was based on just 75 specially selected women in Uppsala, Sweden—a clear case of statistical bias—and each operation was done by gynecologists who worked for Ulmsten, according to investigative journalist Jonathan Gornall.
Johnson & Johnson offered the doctor a $1 million payout if he could come up with even better results on the second round of tests, Gornall reported, citing a licensing agreement contained in court files. Ulmsten came through with a 91 percent success rate.
This second round of studies also involved a small sample: 131 women in six Swedish and Finnish hospitals.
Based on just those two studies and “despite no knowledge of long term outcomes and the compromised nature of the evidence supporting its efficacy, [vaginal mesh implants were] quickly nodded into play by regulatory bodies and rapidly adopted by surgeons around the world,” the BMJ article says.
How Ulmsten, who has since died, was paid without drawing notice was also troubling, the investigation found. He created a company, Medscand, for financial transactions with Ethicon and registered it as the inventor of the implant in regulatory and patent protection paperwork in the United States. In 2001, the U.S. arm of British-based medical device firm Cooper Surgical bought Medscand for $12 million.
Johnson & Johnson confirmed the $1 million payment to Gornall but not the purpose of it, the report says. The company said it “paid Medscand a total of $24,525,000 to purchase all assets associated with the TVT [tension-free vaginal tape] business."
Earlier this year, Johnson & Johnson put out a statement through the Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction that said the mesh had been independently tested:
<p>“In the 20 years that have passed since the study was published in 1998, hundreds of clinical studies with no connection to Dr Ulmsten or Ethicon, including over 100 randomised, controlled trials, have evaluated the clinical performance of TVT, further validating its safety and effectiveness. Scientists from around the world who have conducted and reviewed independent research on pelvic mesh agree it is an important treatment option for women [and] several medical societies comprised of physicians practising in the field of female pelvic medicine have published position statements recognising mid-urethral slings (such as TVT) as the gold standard for the treatment of stress urinary incontinence.”</p>
Before vaginal mesh, the surgical treatment for female urinary incontinence was colposuspension, in which the “neck” of the bladder is picked up to squeeze the urethra, then attached to the pelvic bone—a painful operation that often requires days in the hospital.
Ulmsten’s outpatient procedure bypassed these issues by snaking the plastic “hammock” in through the vagina to shoulder the urethra, using just a local anesthetic.
But as the procedure’s popularity grew, so did reports of problems. Women complained about debilitating chronic pain, painful sex, and genital tearing, and there was an international outcry against Johnson & Johnson. By 2014, women had brought nearly 60,000 lawsuits worldwide.
Earlier this week, the Australian government apologized to affected women and promised regulation overview. Two days before the BMJ report came out, Britain’s National Institute for Health and Care Excellence imposed tighter restrictions on who can get an implant.
In 2016, the FDA backpedaled on its approval of vaginal mesh implants, reclassifying it as a "high-risk medical device" but not banning it or outright questioning its safety. And last year, the FDA determined polypropylene resin, a material commonly found in water bottles used to create many meshes, was safe and effective.
In August, the FDA issued a statement that said products used to treat prolapse like the vaginal mesh implant were associated with serious complications that were not rare and questioned whether it had better outcomes than colposuspension.
Earlier this year, a New York Times investigation highlighted doctors a new issue related to vaginal mesh: doctors were urging women who had not experienced side effects to get them removed in procedures that sometimes resulted in injury but earned the physicians hefty fees.